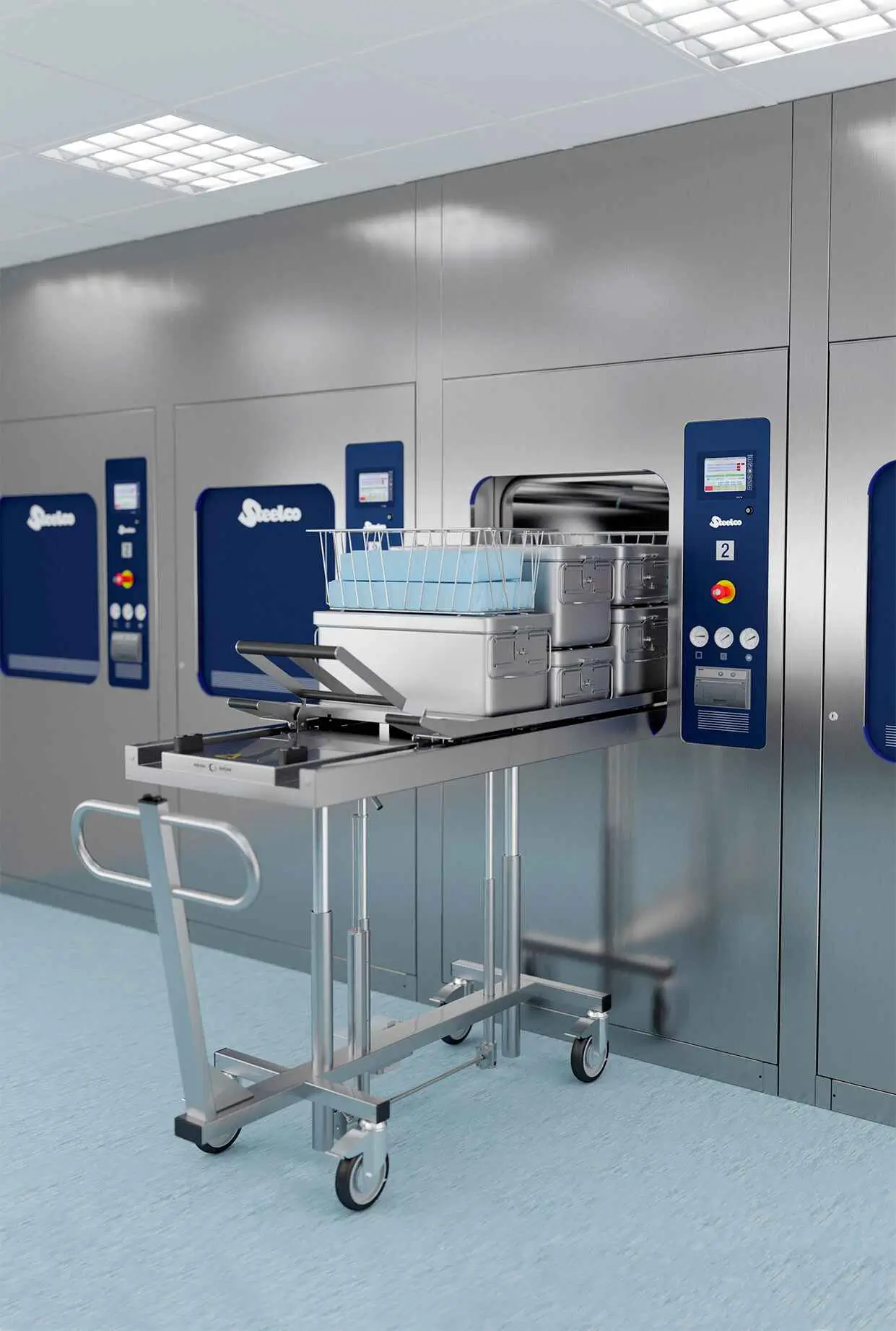
DS Optima PH Parts Washer









Key Features
-
Compact & Cost-Effective
Designed for maximum cleaning power with minimal space requirements and fast-track delivery timelines.
-
GMP-Compliant
Fully aligned with global GMP standards including cGMP, FDA 21 CFR Part 11, and EU Annex 11.
-
Unrivalled Cleaning & Drying
Validatable results with a high-performance hydraulic system and variable temperature-controlled drying.
-
Self-Cleaning System
Prevents cross-contamination with fully integrated chamber and pipework cleaning at every cycle stage.
-
Flexible Configuration
Available in single-door or passthrough double-door with left or right technical area placement.
-
Easy Maintenance & Remote Support
User-accessible components, non-proprietary parts, and VPN-enabled diagnostics reduce downtime and support costs.
-
Custom Racking & Cycle Development
Tailored racks and recipes for deep, uniform cleaning and drying of every item and cavity.
Certifications
- Designed and validated according to current Good Manufacturing Practices (cGMP) | Fully compliant with FDA 21 CFR Part 11 | Developed according to ISPE GAMP© guidelines | Uses FDA-approved (21 CFR Part 177) gaskets and materials
Configurations
Chamber Volume (L) | Overall Dimensions (mm) | Doors | Material | Surface Finish | Drying System | Enquire |
|---|---|---|---|---|---|---|
| 1200L | 2200x1150x2500mm | Single or passthrough hinged glass | AISI Stainless Steel (electropolished) | Ra<0.51 µm (Ra < 0.38 µm upon request) | HEPA filtered air with variable temperature control |
Thank you for requesting a quote. A member of our team will be in touch shortly.

Thank you for requesting a quote. A member of our team will be in touch shortly.