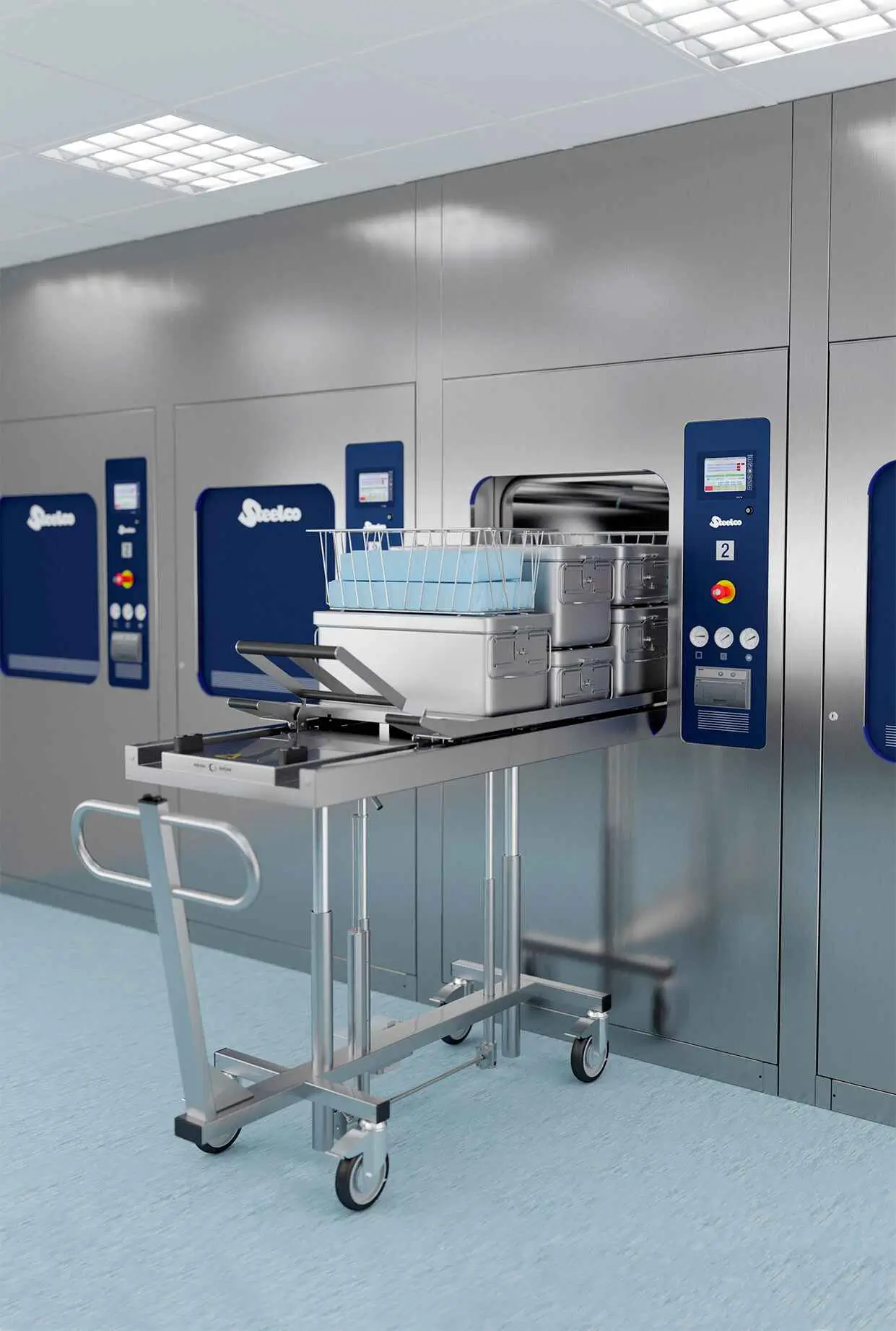
VS G2 & VS Series Steam Sterilisers
A high-quality AISI 316 L squared design chamber minimises dead space for optimal loading and throughput. The best possible jacket covering ensures fast heating and reduces cycle times and areas of potential condensation. High-quality AISI 316 L steam process circuits guarantee long-term reliability and steam cleanliness. Advance Eco Options guarantees unrivaled levels of efficiency, energy, and water consumption reduction with the lowest operating costs per load, assisting our customers in their goals toward environmental sustainability.


















Key Features
-
Flexible Solutions
Five models with vertically sliding doors, with five chamber sizes ranging from 322 to 966 liters / 11.37 to 34.11 cu ft. Five models with horizontal sliding doors, five-chamber sizes, and a book from 530 to 1538 liters / 18.72 to 54.31 cu ft.
-
Process Quality Focus
A series of advanced process safety and quality features, such as a direct steam injection into gaskets, jacket, and chamber; a high-efficiency heat transfer jacket system or the built-in degassing device.
-
Optimised Process Speed
The two-stage liquid ring vacuum pump ensures faster processes and perfectly matches the chamber size. Vacuum pump options and Eco options also help to shorten process times.
-
Ergonomics
Fixed or adjustable transport trolley from 4 up to 12 STU capacity, also on floor loading version.
-
Full Traceability
Full history cycle traceability, compatible with Steelco Data Pro. Ethernet Port for network communication.
-
7″ or 10″ User-Friendly HMI
A sizeable intuitive touchscreen control panel supports operators in every step of their interaction—hands-free cycle programs selection via a barcode scanner.
Certifications
- Steelco VS G2 Series sterilisers comply with the European Regulation for Medical Devices: MDR 2017-745-EU and its revised versions; European Directive for Medical Devices (93/42/EEC and its revised versions); Pressure Equipment Directive (PED 2014/68/EU).
- Steelco VS SC G2 Series sterilisers comply to the European Regulation for Medical Devices: MDR 2017-745-EU and its revised versions; Pressure Equipment Directive (PED 2014/68/EU).
- Steelco VS Series sterilisers conform to European Directive for Medical Devices (93/42/EEC and its revised versions); Pressure Equipment Directive (PED 2014/68/EU).
Configurations
Model | Width (mm) | HMI | Doors | Chamber Volume (L) | Load Capacity (STUs) | Enquire |
|---|---|---|---|---|---|---|
| VS G2 | 950 | Top of Chamber | Vertical Sliding | 996 | 4 – 6 – 8 – 10 – 12 | |
| VS SC G2 | 1250 | Side of Chamber | Vertical Sliding | 966 | 4 – 6 – 8 – 10 – 12 | |
| VS H G2 | 1660 | Side of Chamber | Horizontal Sliding | 1538 | 6 – 9 – 12 – 15 – 18 | |
| VS 3 | 1000 | Side of Chamber | Vertical Sliding | 300 | 3 | |
| VS 4 | 1250 | Side of Chamber | Vertical Sliding | 333 | 4 | |
| VS 6-12 | 1100 | Side of Chamber | Vertical sliding | 910 | 6 – 8 – 10 – 12 | |
| VS H | 1860 | Side of Chamber | Horizontal Sliding | 1344 | 12 – 15 – 18 |
Thank you for requesting a quote. A member of our team will be in touch shortly.

Thank you for requesting a quote. A member of our team will be in touch shortly.